Home

With the resources of the SUNY Research Foundation, and our history of successful partnerships, we are here to help move biomedical products and ideas to market.

Our scientists and core facilities can help move discoveries into practice and technologies into the marketplace.

Upstate is home to top research facilities with highly specialized equipment and advanced instrumentation, to support research and product development.

We are here to create the relationships and partnerships needed to move innovative ideas forward.
Upstate Biotech Ventures
In a partnership between Empire State Development, Upstate Medical University, the SUNY Research Foundation, and Excell Partners, the newly-launched Upstate Biotech Ventures invests in high-potential startups and small businesses affiliated with Upstate Medical University to drive research and technology innovation.
Recent Tech from SUNY Upstate
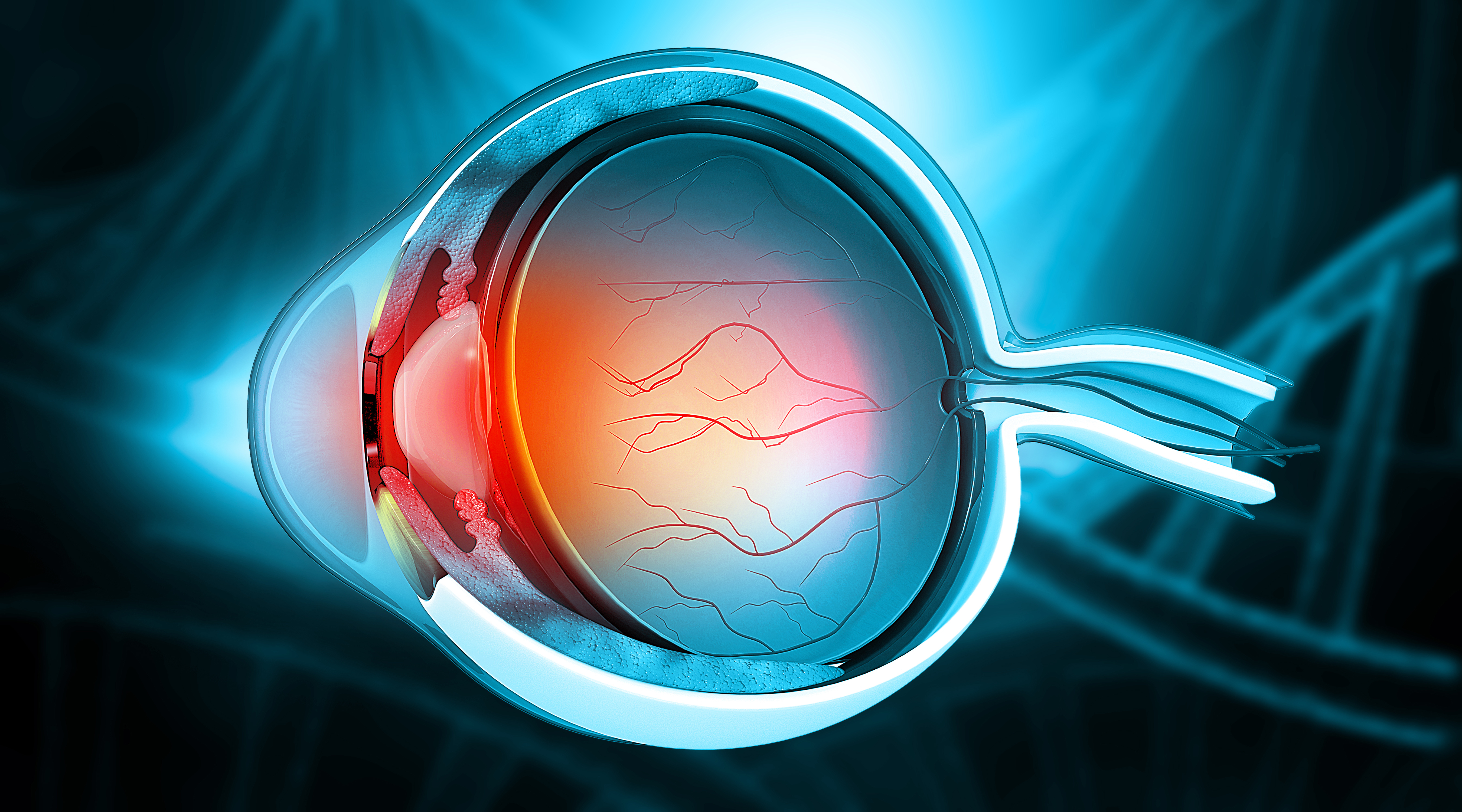
Novel tools that promote the delivery of therapeutics to treat inner retinal diseases such as diabet...
Novel tools that promote the delivery of therapeutics to treat inner retinal diseases such as diabetic retinopathy, retinal vein occlusion, and retinitis pigmentosa. Background:
Non-surgical access to tissues for direct biological manipulation, e.g. gene transfer, stem cell transplantation, and drug delivery, can be difficult because many tissues and organs are surrounded with, and protected by, a nearly impenetrable barrier called the basement membrane (BM). In the eye, the inner limiting membrane (ILM) is a special BM that prevents movement into the retina from the vitreous. Current methods to circumvent the ILM involve its physical or enzymatic removal and have limited efficacy. As a result, successful cell- and gene- based therapies have targeted a subretinal approach, which has proven effective for the outer retina, but is of extremely limited use for the inner retina. Technology Overview:
This technology describes the use of Ntn4-based molecules to disrupt the laminin polymer needed to form the ILM, allowing for access to these otherwise inaccessible tissues, and for therapies based on that access. This approach is also applicable to a wide variety of other tissues that contain a basement membrane that block the delivery of therapeutic agents. Of particular interest are organs with hard-to-breach BMs, including the kidney glomerulus, cornea and lens capsule, and the dermal/epidermal junction of the skin. https://suny.technologypublisher.com/files/sites/adobestock_4964568011.jpegAdvantages:
• Provides non-surgical access to inner retinal tissues.
• Promotes gene-based and cell-based therapeutics in inner retinal diseases.
• Applicable to a wide variety of other tissues. Applications:
Manipulate the molecular structure of the ILM to promote integration of neural stem cells, viral transfection, or small molecule delivery for the treatment of diabetic retinopathy, retinal vein occlusion, and retinitis pigmentosa and other inner retinal diseases. Intellectual Property Summary: Provisional patent application filed: 63/709,982Stage of Development:
TRL 3 – Experimental proof of concept Licensing Status:
This technology is available for licensing.Licensing Potential:
This technology would be of interest to entities involved in gene therapy, stem cell therapy, and targeted drug delivery. Potential licensees include biopharmaceutical companies, ophthalmology-focused biotech firms, regenerative medicine developers, and platform technology companies seeking to enhance delivery of biologics or cell-based therapies across tissue barriers
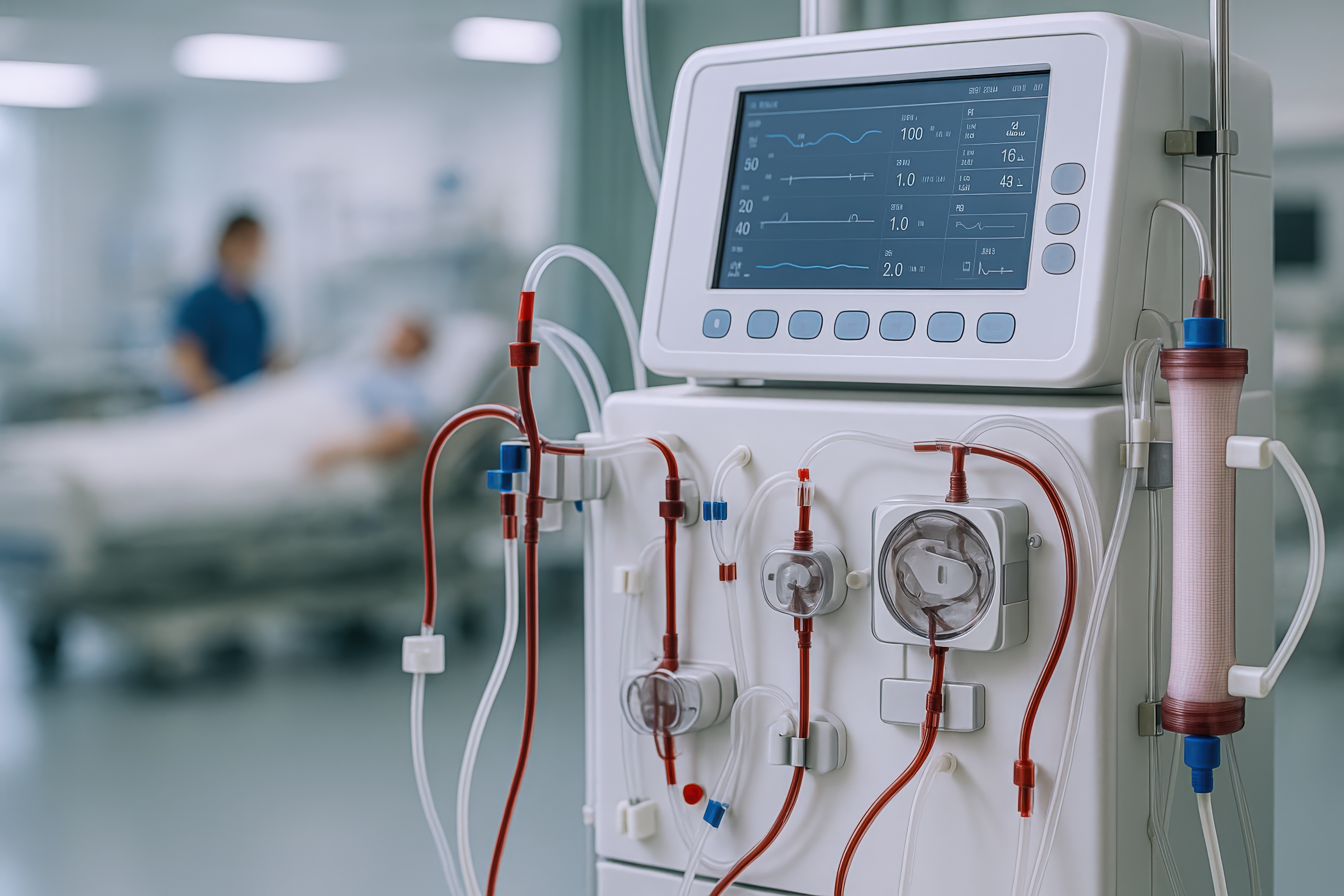
This device uses ultrasonic vibrations delivered through a specialized wire to safely and efficientl...
This device uses ultrasonic vibrations delivered through a specialized wire to safely and efficiently clear blockages or remove implants from medical tubes, offering a non-invasive, customizable solution for maintaining and treating tube obstructions in healthcare settings. Background:
Nephrostomy tubes and other indwelling medical devices are critical for managing renal obstructions and maintaining urinary drainage in patients with compromised kidney function. However, these tubes are highly susceptible to clogging due to the accumulation of biofilm, mineral deposits, and debris. Such blockages can lead to infection, loss of tube function, and even life-threatening complications if not addressed promptly. Current clinical practice often involves routine tube exchanges, emergency interventions, or flushing procedures to maintain patency, all of which can be uncomfortable for patients, resource-intensive for healthcare systems, and disruptive to patient care. The need for a rapid, effective, and minimally invasive solution to manage and prevent tube occlusion is therefore significant, with the potential to improve patient outcomes and reduce healthcare costs. Existing approaches to clearing clogged nephrostomy tubes and similar devices are fraught with limitations. Flushing and wire probing are frequently ineffective against stubborn obstructions like calcifications or dense biofilms and may require multiple attempts or specialized equipment. Tube exchanges and surgical interventions, while more definitive, are invasive, carry procedural risks, and often necessitate hospital admission or specialized staff, leading to delays in care and increased burden on healthcare resources. Furthermore, these methods do not address the underlying tendency for tubes to re-occlude, resulting in repeated interventions over time. There is a clear unmet need for a solution that can be rapidly deployed at the bedside or in outpatient settings, offering reliable, non-invasive clearance of obstructions without the drawbacks of current techniques.Technology Overview:
This technology is an ultrasonic transmission system specifically engineered for medical applications such as clearing obstructions from nephrostomy tubes and mobilizing retained implants. The system converts electrical energy into ultrasonic vibrations at adjustable frequencies, paired with an ultrasonic horn and replaceable cavitation wire. The modular design allows for various wire diameters, enabling customization for different clinical scenarios and types of obstructions. What differentiates this solution is its unique integration of modularity, medical-specific enhancements, and procedural flexibility into a single system. Unlike traditional methods that often require invasive procedures, specialized staff, or hospital admission, this device provides a rapid, non-invasive alternative that can be used at the bedside or potentially in home settings. By leveraging commercially available ultrasonic components and introducing novel, application-specific enhancements, the system delivers superior energy transfer and cavitation effects tailored for medical use. This results in reduced hospital resource utilization, extended tube lifespan, and improved patient outcomes, setting it apart from existing solutions in both efficacy and versatility. https://suny.technologypublisher.com/files/sites/adobestock_1641364005.jpegAdvantages:
• Non-invasive and rapid clearance of obstructions in medical tubes, reducing the need for surgical interventions.
• Customizable ultrasonic frequency and power settings for tailored clinical applications.
• Modular, replaceable cavitation wire with biocompatible materials and surface modifications enhances cavitation and mechanical disruption.
• Optimized ultrasonic horn design ensures efficient energy transfer and resonance tuning for improved performance.
• Extends the lifespan of nephrostomy tubes and other medical implants by preventing clogging and biofilm buildup.
• Enables bedside or potential home use, decreasing hospital admissions and healthcare resource utilization.
• Adaptable to a variety of medical tubes and retained implants, including ureteral stents and catheters. Applications:
• Clearing clogged nephrostomy tubes
• Removing biofilm from catheters
• Mobilizing retained ureteral stents
• Fragmenting urinary tract stones
• Clearing obstructions in medical drains Intellectual Property Summary:
Patent PendingStage of Development:
TRL 3Licensing Status:
This technology is available for licensing.
The Modular Hybrid Simulator is an advanced training platform for endovascular procedures, combining...
The Modular Hybrid Simulator is an advanced training platform for endovascular procedures, combining patient-specific 3D modeling with real-time operative feedback to enhance learning and procedural planning. Background:
Endovascular training currently faces challenges such as inconsistent learning curves among trainees and the limitations of existing simulation models, which are often static and not patient-specific. Traditional methods lack the adaptability and detailed feedback required to effectively improve procedural skills and decision-making. Additionally, the rise of robotic automation in endovascular practices necessitates training tools that emphasize human judgment and ensure operator proficiency. These issues prompted the development of a more dynamic, customizable simulator that integrates real patient data for more realistic and effective training experiences.Technology Overview:
The Modular Hybrid Simulator is a comprehensive platform designed to replicate endovascular procedures with high fidelity. It leverages 3D auto-segmentation software to process patient data obtained from CT angiography, generating accurate, patient-specific vascular models. These models serve both educational and procedural planning purposes, allowing trainees to engage with realistic anatomical scenarios. Embedded sensors within the simulator provide real-time operative feedback, enabling users to track and improve their procedural techniques. This feedback supports gamification elements, making the learning process more engaging and measurable. What distinguishes this simulator is its modular design, which allows customization of simulation scenarios based on individual patient anatomies and procedural complexities. This flexibility overcomes the limitations of static models and supports a wide range of training needs from novice to expert levels. Furthermore, the system captures detailed data on operative techniques, offering insights that can be utilized to refine future robotic automation technologies in endovascular treatment. By combining realistic anatomical modeling, dynamic feedback, and data capture, the simulator not only enhances skill acquisition but also contributes to advancing the safety and efficacy of both manual and robotic endovascular interventions. https://suny.technologypublisher.com/files/sites/adobestock_967369379.jpegAdvantages:
• Utilizes patient-specific anatomical data for more realistic and effective training scenarios.
• Embedded sensors provide real-time feedback and performance tracking to enhance learning.
• Gamification of the training process increases engagement and skill retention.
• Collects operative data to support the safe integration of robotic technologies. Applications:
• Educational tool for medical students and specialists training in endovascular procedures.
• Pre-procedural planning using patient-specific models to improve clinical outcomes.
• Skill assessment and improvement through performance tracking and gamified feedback.
• Research and development platform for advancing robotic-assisted endovascular technologies. Intellectual Property Summary:
Patent PendingLicensing Status:
This technology is available for licensing.

PepTelligence is an innovative computational tool designed to evaluate and optimize therapeutic pept...
PepTelligence is an innovative computational tool designed to evaluate and optimize therapeutic peptides by integrating multiple biochemical and pharmacological factors into a unified scoring system. Background:
The development of PepTelligence addresses a significant challenge in peptide drug discovery: the absence of a comprehensive evaluation system that can simultaneously assess various important aspects of therapeutic peptides. Traditional methods often examine individual properties in isolation, which can lead to incomplete or inefficient optimization processes. This need became apparent during research focused on RNA Polymerase I protein–protein interactions and inspired the creation of a generalized approach for peptide assessment.Technology Overview:
PepTelligence operates as a computational platform that consolidates multiple criteria—such as biochemical features, structural characteristics, interaction profiles, and pharmacological safety—into a single predictive score for therapeutic peptides. By integrating these diverse data points, the tool provides a holistic measure of a peptide’s drug-likeness, facilitating informed decisions on which candidates to advance or improve. The system's adaptability allows it to handle a wide range of peptide types and therapeutic targets, making it valuable across different stages of drug development. Unlike existing tools, PepTelligence does not rely on third-party code and is built upon original algorithms developed by its creators. This proprietary design ensures a unique and targeted approach to peptide analysis. The software’s capacity to pinpoint specific areas for enhancement assists researchers and developers in refining peptides to achieve optimal efficacy and safety profiles. https://suny.technologypublisher.com/files/sites/adobestock_685417441.jpegAdvantages:
• Comprehensive Evaluation: Integrates multiple biochemical, structural, and pharmacological criteria into a single cohesive score.
• Predictive Power: Accurately forecasts peptide drug-likeness to guide development decisions.
• Adaptability: Suitable for diverse therapeutic peptides and drug discovery contexts.
• Proprietary Design: Developed without third-party code, offering a unique, tailored analysis platform.
• Optimization Guidance: Identifies specific properties for improvement, enhancing drug development efficiency. Applications:
• Pharmaceutical companies engaged in peptide-based drug development and optimization.
• Academic research laboratories studying therapeutic peptides and protein interactions.
• Biotechnology firms focusing on novel peptide therapeutics and personalized medicine.
• Drug discovery pipelines requiring integration of multiple evaluation criteria for peptide candidates. Intellectual Property Summary:
Patent PendingStage of Development:
TRL 2Licensing Status:
This technology is available for licensing.




